Okur Research Group Publishes at The Journal of Physical Chemistry Letters
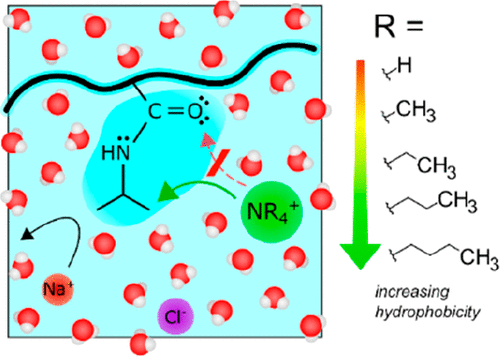
The presence and nature of ions influence the solution properties of macromolecules. Although anions have been heavily investigated, the cationic effects have been mostly considered as simple exclusion or very weak interaction with neutral surfaces. In the recent J. Phys. Chem. Lett. article, Okur Research Group have systematically studied the influence of hydrophobic tetraalkylammonium cations on model poly(N-isopropylacrylamide) (PNIPAM) macromolecule in aqueous solutions. Employing a multi instrumental approach including macromolecule solubility,1H NMR and ATR-FTIR measurements, a direct binding between the greasiest cations and the isopropyl group of the macromolecule was revealed. Moreover, the amide oxygen was shown not being the main direct-binding site. These results clearly exhibited that the most weakly hydrated, hydrophobic cations can bind to neutral macromolecules with a similar strength to the weakly hydrated Hofmeister anions. You can read more by clicking here.